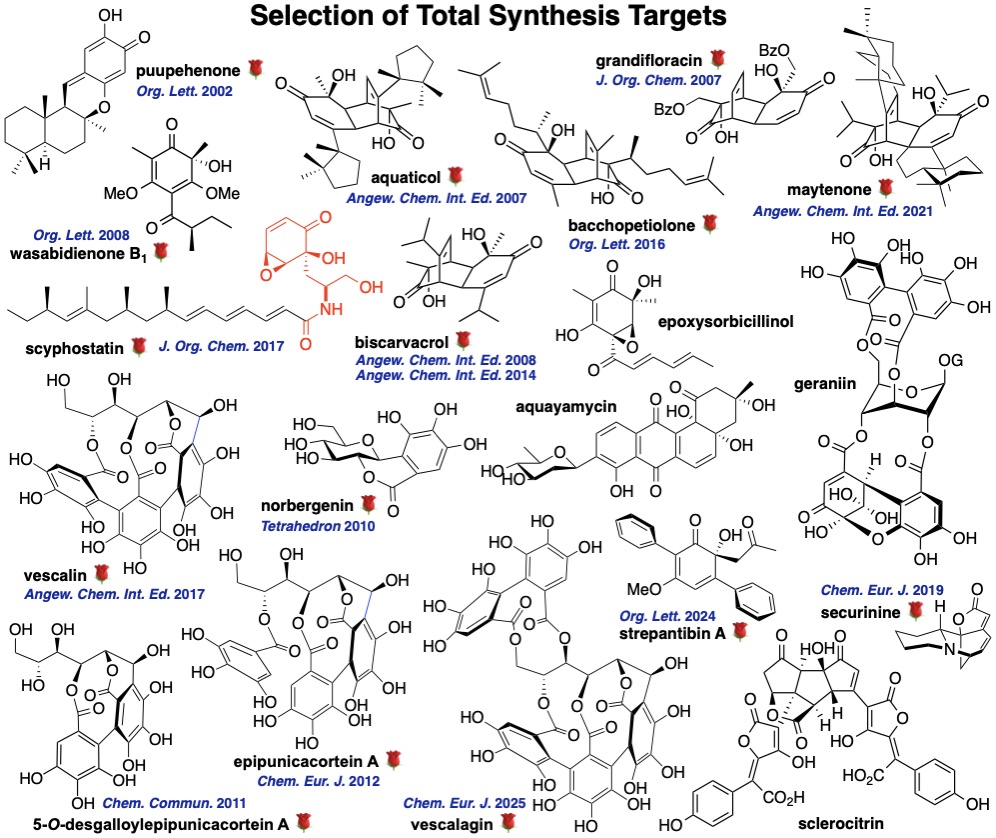
Our selection of natural products is driven by both their structural peculiarities and their presumed biological effects, as well as by the aim of illustrating the value of the synthesis methodologies we are developing, such as the iodane-mediated regio- and stereoselective oxygenative phenol dearomatization reactions. Members of the terpenoid, alkaloid, polyketide and plant polyphenol families of natural products have thus been, still are and will be the targets of our total synthesis efforts, whose success often relies on bio-inspired routes.